QVF® Bromine Processes
- Debromination of brines down to 10ppm bromine -



HIGHLIGHTS
- Production of 99,9% bromine from brines
- Debromination of brines down to 10 ppm Bromine
- Proven and state of the art process know how
- Safe equipment especially developed for the bromine process
- Glass and glass-lined steel as ideal highly corrosion and diffusion resistant material
- Plants as per highest international and local safety standards
- Numerous reference bromine plants around the world
- Bromine experience since more than 50 years
- Safe bromine process and reliable equipment from one responsible partner
Use of Bromine
Bromine is widely used in the chemical and pharmaceutical industry. Major applications are brominated organic components used as
- Flame retardants e.g. polybrominated biphenyls
- Pesticide e.g. methyl bromide
- Reactive intermediates in organic synthesis and pharmaceutical ingredients
Flame retardant

Empty line to generate FULL WIDTH
Pesticides

Reactive intermediates

Sources of Bromine
Natural sources for bromine are chloride sources such as
- natural salt stocks
- seawater
where bromides are accompanying the chlorides.
Industrial sources for bromine recovery are
- quench waters of waste incinerators (coming from e.g. the flame retardants)
- effluents of industrial bromination processes
since the reactions to form the a.m. chemicals may generate hydrogen bromide as a by-product:
R-H+Br2 => R-Br+HBr
Clearly, in this case no more than half of the bromine added ends up in the product - the rest being rejected as hydrogen bromide gas and as dissolved bromides (often rejected in washing solutions).
Bromine is an expensive, widely used raw material, and hence suggests that it should be recovered.
Quench waters from waste incinerator

Seawater from solar evaporation ponds

Safety first
Bromine is highly corrosive, toxic and causes serious chemical burns. 3ppm in the air are immediately dangerous to life and health. Therefore, it is vitally important to take highest safety precautions when working with bromine.
Accordingly, plants processing bromine have to be made of highly corrosion and diffusion resistant and robust materials. QVF® borosilicate glass 3.3 and De Dietrich® glass-lined steel are therefore ideal materials for their construction. All measures are taken to avoid any bromine leakage – e.g. the tightness of the flange connections is certified according to the German regulation TA-Luft. The equipment fulfills the requirements of the European Pressure Directive. The vent systems are, as a matter of course, equipped with bromine scrubbers.
The material quality, the adequate detailed design and the reliable process are the bases for a safe handling of the bromine. To this end, it is especially advantageous to get the components, the equipment and the process from one single experienced manufacturer.
Highly corrosion resistant piping for bromine plants

Treating Bromide Containing Brines
There are two different objectives when treating bromide containing brines. One objective is the production of bromine and the other one is to debrominate the brine to get rid of the bromides. The production of bromine from brines as well as the debromination of brines is based on the oxidation of bromide to bromine. In the laboratory scale as oxidating component concentrated sulfuric acid or acidified KMnO4 is used. In industrial scale mainly chlorine, but also hydrogen peroxide is used.
Bromine production plant

Bromine scrubber

.
Production of Bromine
The production of bromine from brines becomes economically advantageous when the bromide concentration is more than 2,5g/l. If seawater is used, it will have to be pre-concentrated, mostly by solar evaporation ponds, to reach that concentration. The production of bromine is done in 2 steps. In the first step the bromides are oxidized with chlorine
2Br- + Cl2 => 2Cl- + Br2
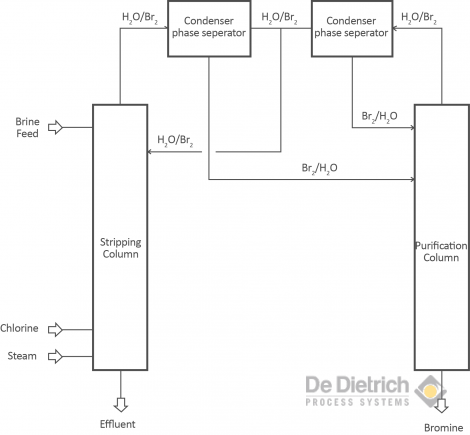
and the bromine is stripped with steam in the same column. The second step is the purification of the bromine from water and chlorine by rectification in a separation column. These steps are carried out in columns interlinked as shown in the block scheme A. The purification is eased by the fact that bromine has a low solubility in water (only 28g-Br2/1000g-H2O) so that efficient phase separators can be used. Purification is realized by separating the chlorine and water from the bromine with a rectification column. Pure bromine (99,9%) can be withdrawn at the bottom of the thermal separation column.
Block scheme A - Bromine production
Debromination
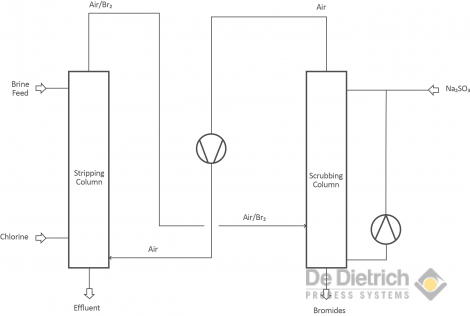
Bromide impurities in chloride brines can reduce the quality of the products produced from chloride solutions, and can also hamper the process handling the chlorides. The QVF debromination process can reduce the bromide content in brines down to 10ppm. The process shown in block scheme B is also based on the selective oxidation of bromides, which can be done either with chlorine or with hydrogen peroxide. Stripping of the bromine is done with recycled air instead of steam, and is also called “cold debromination”. If elementary hazardous bromine is not desired, it is reduced by e.g. sodium sulfite back to harmless bromide in a second absorption column.
Br2 + Na2SO3 + H2O => 2HBr + Na2SO4
Block scheme B - Debromination
Bromine Process Expertise
In order to make these plants most efficient, the columns and their internals have to be highly corrosion resistant and specifically constructed for the process. Their task is to equally distribute large feed streams and to redistribute them excellently along the column to avoid dead volumes and to maximize the mass transfer area. This can be realized, for example, by our patented QVF CORE-Trays which ensure a maximum contact of the liquid phase with the gas phase generated e.g. by our structures packing DURAPACK® made of borosilicate glass 3.3. The bromine is also safely handled by our corrosion resistant QVF shell and tube heat exchangers with tubes either made of borosilicate glass 3.3. or SiC. The proven bromine evaporators are made of tantalum.
Whilst the above described processes are extremely comprehensive, the solution is not always simple since feeds are often contaminated with organic materials e.g. from the reactors. They have to be removed by steam stripping before entering the stripping column since they may react with bromine and consequently reduce the yield. Even in such difficult cases, the wide experience that exists within De Dietrich Process Systems coupled with our extensive pilot plant facilities in Germany, allow us to find the right solution to the problem of removing organics and increasing the yield to provide you with the ultimate process design.
De Dietrich Glass-lined reaction column

QVF Reaction column DN1000 made of borosilicate glass 3.3

Highlights
- Production of 99,9% bromine from brines
- Debromination of brines down to 10 ppm Bromine
- Proven and state of the art process know how
- Safe equipment especially developed for the bromine process
- Glass and glass-lined steel as ideal highly corrosion and diffusion resistant material
- Plants as per highest international and local safety standards
- Numerous reference bromine plants around the world
- Bromine experience since more than 50 yeras
- Safe bromine process and reliable equipment from one responsible partner
