Hydrochloric Acid Treatment

Hydrochloric acid is one of the commonest chemicals available today and is used in a wide variety of applications, some of the most important being:
- Chemical synthesis - organic chlorides for agrochemicals and polymers as PVC
- Production of inorganic chemicals for water treatment
- Regenerations of ion exchangers
- Metal cleaning - pickling acids
- Petrochemical industry - activation of oil wells
- Food industry - production of e.g. sugars, glutamates and starch syrups
- Leather/tanning industry
In many of these applications, contaminated aqueous HCI is discharged as a by-product (spent resp. diluted acid), but due to the low cost and availability of fresh supplies, little or no effort has been expended in purifying the contaminated aqueous HCI for re-use. We provide konw-how and plants to recover, purify and concentrate hydrochloric acid even above the azeotropic point.
On the other hand hydrogen chloride gas is generated as a by-product or waste gas by a variety of processes within the process industries. In many countries there are strict maximum emission levels for hydrogen chloride which mean that it must be removed from waste gas streams. We provide plants scrubbing hydrochloric acid out of the waste gas, or where the concentration is high enough, absorbing HCl to produce a hydrochloric acid as a product.
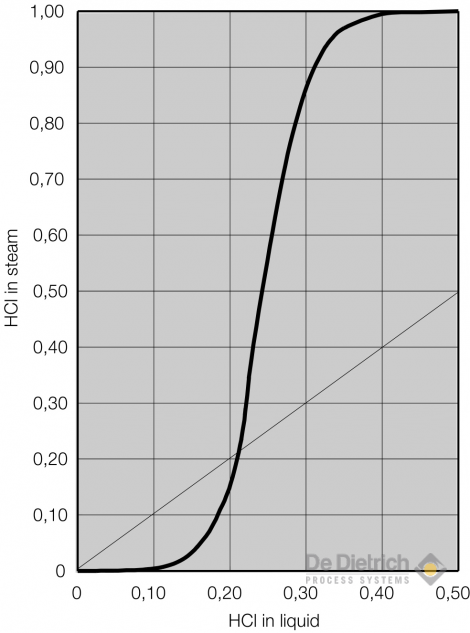
Hydrochloric acid in water
HCl is very good soluble in water. The below diagramm shows the HCl-ratio in the vapour phase to the liquid phase at atmospheric boiling conditions.
Up to a concentration of ~10wt% HCl in water the vapour pressure of HCl is down to some ppm so that scrubbing of HCl out of waste gases is easily possible. The HCl vapour pressure is then increasing drastically with the concentration in the liquid phase.
Aleady at a concentration of 21wt% HCl the concentration in the vapour phase is the same as in the liquid phase reflecting the azeotrop point of this binary mixture. A concentration of diluted hydrochloric acid above this azeotropic point cannot be realised with a simple rectification and is described seperatly.
At a concentration of ~40wt% HCl in the liquid phase the vapour consist almost of only HCl, means that this is the maximum concentration of HCl in water at atmospheric pressure.
Equilibrium Diagram for HCl/Water